UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
|||
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On June 7, 2022 at 12:40 EDT, SAB Biotherapeutics, Inc.’s Chief Operating Officer, Christoph Bausch, Ph.D., MBA, will give a presentation at the Large Animal Genetic Engineering Summit ("LAGE") as part of the summit’s “Gene Editing to Improve Human Health” track.
The presentation, titled “Leveraging Genetically Engineered Ungulates to Produce Novel Human Biotherapeutics,” will highlight SAB’s novel immunotherapy platform of Transchromosomic (Tc) Bovine™ (genetically engineered cattle) that can consistently and reliably produce fully human antibodies without the need for convalescent plasma from human donors. Dr. Christoph Bausch will introduce a range of topics, including an overview of SAB’s DiversitAb™ platform centered around Tc Bovine and an overview of SAB’s pipeline programs that include SAB-185, the company’s anti-SARS-CoV-2 therapeutic; SAB-176, the company’s seasonal influenza therapeutic; and SAB-142, the company’s Type 1 diabetes and organ transplantation therapeutic. Additionally, he will discuss the potential that SAB’s novel immunotherapy platform has to expand into personalized medicine through the development of Transchromosomic (Tc) Goats™.
A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The foregoing (including Exhibits 99.1) is being furnished pursuant to Item 7.01 and will not be deemed to be filed for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise be subject to the liabilities of that section, nor will it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act.
Cautionary Note Regarding Forward-Looking Statements
Certain statements made herein that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, including the development and efficacy of SAB-185, our influenza program and other discovery programs, our cash runaway into 2023 and potential future government and third-party collaborations or funded programs.
These statements are based on the current expectations of SAB and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on, by any investor as a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict, will differ from assumption and are beyond the control of SAB. A further description of risks and uncertainties can be found in the sections entitled “Risk Factors” in SAB’s Annual Report on Form 10-K, quarterly reports on Form 10-Q, and other periodic reports filed with the Securities and Exchange Commission and available at https://www.sec.gov/
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits. The exhibits listed on the Exhibit Index are incorporated herein by reference.
Exhibit Number |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File-the cover page XBRL tags are embedded within the Inline XBRL document. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
SAB Biotherapeutics, Inc. |
|
|
|
|
Date: |
June 7, 2022 |
By: |
/s/ Eddie J. Sullivan |
|
|
|
Eddie J. Sullivan |

Large Animal Genetic Engineering Summit, June 2022 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL Leveraging Genetically Engineered Ungulates to Produce Novel Human Biotherapeutic

Forward Looking Statements 2 The material in this presentation has been prepared by SAB Biotherapeutics, Inc. (SAB) and is general background information about SAB’s activities current as of the date of this presentation. This information is given in summary form and is not intended to be complete. Information in this presentation, including financial forecasts, should not be considered advice or a recommendation to investors or potential investors in relation to holding, purchasing or selling securities or other financial products or instruments and does not take into account any particular investment objectives, financial situation or needs. This presentation may contain forward looking statements including statements regarding our intent, belief or current expectations with respect to SAB’s businesses and operations, market conditions, results of operations and financial condition, capital adequacy, specific provisions and risk management practices. Readers are cautioned not to place undue reliance on these forward-looking statements. SAB does not undertake any obligation to update any information herein for any reason or to publicly release the result of any revisions to these forward-looking statements to reflect events or circumstances after the date hereof to reflect the occurrence of unanticipated events. While due care has been used in the preparation of forecast information, actual results may vary in a materially positive or negative manner and the presentation may contain errors or omissions. Forecasts and hypothetical examples are subject to uncertainty and contingencies outside SAB’s control. Past performance is not a reliable indication of future performance. Unless otherwise specified, information is current at the date hereof, unless specifically noted. © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Novel DiversitAb™ Platform for Developing Highly-Differentiated Immunotherapies Vertical integration enables rapid, scalable development of multi-targeted products Robust, growing clinical-stage pipeline spanning multiple therapeutic areas Established proof-of-concept through US Government funded programs & partnerships totaling ~$200MM Strong corporate position with experienced leadership team and growing infrastructure Leveraged advanced genetic engineering & antibody science to develop Tc bovine-derived fully-human polyclonal antibodies © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL Innovative DiversitAb™ platform produces a new class of targeted fully-human, highly-potent polyclonal antibodies

DiversitAb™ Proprietary Platform Technology © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Only transgenic animal that carries the entire human immunoglobulin (Ig) heavy and light (κ) chain loci. HAC is subject to mitosis along with the other 46 chromsomes. HAC present in the Tc Bovine allows for the highest production of human antibody repertoire most similar to humans. Tc Bovine Tc Bovine™ contain all the human immunoglobulin genes Human artificial chromosome (HAC) contains the entire human immunoglobulin loci (IgH + Igκ) © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL A Natural Way to Produce Human Polyclonal Antibodies

Human Antibody Production in Bovine B-Cell bIg Bovine chromosomes X X Bovine DNA-binding proteins Tc Bovine B-cells Remove species-incompatibility in protein-protein interaction Remove species-incompatibility in protein-DNA interaction HAC mRNA mRNA Human Bovine Chimeric IgM on B-Cell Receptor Fully-human IgG © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

B-Cells Produce Anti-Target Fully-Human Polyclonal Antibodies Hyperimmunization Multiple immunizations drive titers to extremely high levels with exceptional avidity maturation and potency B-Cells Produce Human Antibodies Natural and somatic mutation drives very high-level B-cell clone avidity maturation in Tc Bovine Therapeutic Diverse mixture of anti-Target human polyclonal antibodies allowing production of a fully-human immunoglobulin (hIgG) Rich diversity of IgG antibodies to Spike protein epitopes Fc binding to FcR ligands allows effector cell recruitment & activates complement Transferred full germline repertoire of human antibody response 7 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL Antigen

FDA: CENTER FOR DRUG EVALUATION & RESEARCH (CDER) FDA: CENTER FOR BIOLOGICS EVALUATION & RESEARCH (CBER) pAbs TARGET Monoclonal Approach Highly-targeted with specific activity Iterative Ab identification and selection process Selected and cloned in vitro May promote escape mutants via selective pressure Resistance may develop as pathogen/target mutates Current cocktail trend to address resistance Polyclonal Approach Diversity of antibodies with multiple modalities Naturally selected and produced in vivo Effective against escape mutants Reduced possibility of resistance Activates cellular immunity Synergistic properties not duplicated by mono- or oligoclonals mAb TARGET Clones of a single antibody bind to a specific epitope Natural mixture of many antibodies bind to multiple epitopes Characterized Monoclonal Antibody Plasma-Derived Polyclonal Antibodies Polyclonals: Broader Spectrum Efficacy Valuable in Range of Indications 8 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

B/Florida/04/2006 Challenge strain SOURCE: NEXTFLU AT HTTPS://NEXTFLU.ORG/VIC/12Y/ Antibodies produced to B/Phuket/3073/2013 protected against B/Florida/04/2006 2006 2017 B/Phuket/3073/2013 Vaccination strain Highly-Mutational Influenza Virus BYAM PHYLOGENIC TREE 100% Protection at All Dose Levels in Influenza Mouse Challenge * x Adaptive & Cross Reactive to Mutating Strains Efficacy Against Mutational Drift 9 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Consistent, Replicable Platform In Vivo Efficacy Demonstrated Across a Broad Range Targets TARGET EFFICACY MODEL(S) COLLABORATORS Anthrax 100% mouse (lethal) Food and Drug Administration Alphaviruses 100% 100% mouse (lethal aerosol) non-human primate (viral clearance) Naval Medical Research Center, University of Pittsburgh, NIH: National Institute of Allergy and Infectious Diseases Clostridium Difficile 100% 87% hamster (lethal) mouse (lethal) Novavax Dengue 100% non-human primate (viral clearance) Naval Medical Research Center Ebola 90% 100% mouse (lethal) non-human primate (lethal) Naval Medical Research Center, NIH: National Institute of Allergy and Infectious Diseases, Novavax Hantavirus 80-100% 100% hamster (lethal) non-human primate (viral clearance) United States Army Medical Research Institute of Infectious Diseases Influenza 100% 100% mouse (lethal) mouse (lethal aerosol) National Institutes of Health, University of South Dakota, Utah State University, Naval Medical Research Center Plague* 100% Mouse (lethal aerosolized) United States Army Medical Research Institute of Infectious Diseases MERS-CoV 100% mouse (viral clearance) Biomedical Advanced Research and Development Authority, Naval Medical Research Center, NIH: National Institute of Allergy and Infectious Diseases, Novavax Zika 100% 100% 100% mouse (lethal) hamster (lethal) non-human primate (viral clearance) Public Health Agency of Canada, Utah State University Harvard University *Current DoD interest © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL 10

CLINICAL TRIAL INDICATION COLLABORATORS Phase 1b Mycoplasma hominis Brigham and Women's Hospital, Harvard Phase 1 MERS-CoV Naval Medical Research Center; NIH NIAID Phase 1 Type A and B Influenza Naval Medical Research Center; University of South Dakota Phase 2a Type A and B Influenza Naval Medical Research Center; University of South Dakota Phase 1 SARS-CoV2 DoD; BARDA; University of Pittsburgh Phase 1b SARS-CoV2 DoD; BARDA; University of Pittsburgh Phase 2 SARS-CoV2 DoD; BARDA; DAIDS NIH NIAID; University of Pittsburgh Phase 3 SARS-CoV2 DoD; BARDA; DAIDS NIH NIAID; University of Pittsburgh Human Clinical Trial Experience Clinical Trial Proof of Concept © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL 11

SAB-185 Anti-SARS-CoV2

SAB-185: Specifically Targeted Human Immune Response Spike Glycoprotein Diversity of antibodies and uniquely combinatorial paratopes drives effector functions including antibody and complement dependent cellular cytotoxicity Multiple blocking and neutralizing antibody species bind to single epitope Multiple blocking and neutralizing antibodies with uniquely determined and multifactorial paratopes bind to single multi-conformational antigen epitope D Spike Glycoprotein Receptor binding domain in S1 spike protein binds to ACE-2 receptor on human cells; then undergoes a conformational change to allow the S2 spike protein domain to fuse with the cellular membrane leading to infection of the cell SAB-185 Polyclonal Spike Protein MOA Antibodies bind multiple conformations of SARS-CoV-2 extracellular spike protein epitope and appears to prevent most all conformations of the infectious determinant spike protein from interacting with ACE-2 receptors on host cells, allowing effector cells to phagocytize virus and eliminate/lyse infected cells via complement MOA of Novel Polyclonal Antibody Raised Against SARS-CoV-2 Spike Protein 13 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Addresses Escape Mutants: SAB-185 Superior to Monoclonal Antibody 14 Selection for VSV-SARS-CoV-2 Wild Type Escape Mutation WASHINGTON UNIVERSITY SCHOOL OF MEDICINE–ST. LOUIS; 15 JAN 2021 Escape Mutants No Escape Mutants SAB-185 Targeted High-Potency Polyclonal Mixture Monoclonal Ab LOT 1 LOT 5 LOT 6 No Antibody 2H04 E484K E484A F486S © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Mutational Drift and Shift of COVID Variants x Haseltine W. Birth Of The Omicron Family: BA.1, BA.2, BA.3. Each As Different As Alpha Is From Delta. Online Forbes Article BA.1 BA.2 BA.3 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-185 Provides 100% protection against Delta and Omicron in an in-vivo hACE2 Hamster model Note: Control demonstrated 100% mortality in the ACE2 Hamster Model against Delta Note: Control demonstrated 0% mortality in the ACE2 Hamster Model against Omicron PERFOMRED BY WILLIAM KLIMSTRA’s LAB AT UNIVERSITY OF PITTSBERGH; hACE2 HAMSTER MODEL DEVELOPED BY WANG LAB AT USU © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL 16
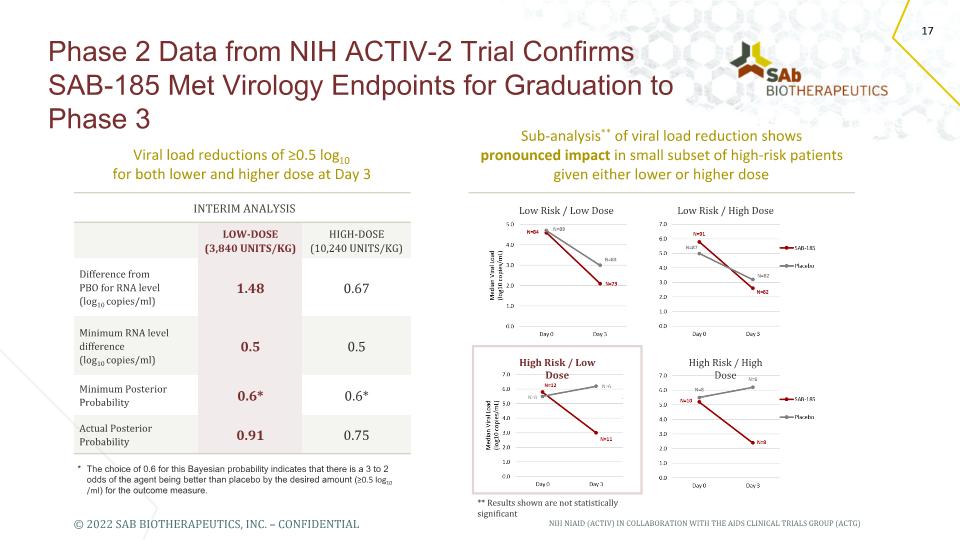
Phase 2 Data from NIH ACTIV-2 Trial Confirms SAB-185 Met Virology Endpoints for Graduation to Phase 3 * The choice of 0.6 for this Bayesian probability indicates that there is a 3 to 2 odds of the agent being better than placebo by the desired amount (≥0.5 log10 /ml) for the outcome measure. INTERIM ANALYSIS Interim Analysis LOW-DOSE (3,840 UNITS/KG) HIGH-DOSE (10,240 UNITS/KG) Difference from PBO for RNA level (log10 copies/ml) 1.48 0.67 Minimum RNA level difference (log10 copies/ml) 0.5 0.5 Minimum Posterior Probability 0.6* 0.6* Actual Posterior Probability 0.91 0.75 Viral load reductions of ≥0.5 log10 for both lower and higher dose at Day 3 Sub-analysis** of viral load reduction shows pronounced impact in small subset of high-risk patients given either lower or higher dose NIH NIAID (ACTIV) IN COLLABORATION WITH THE AIDS CLINICAL TRIALS GROUP (ACTG) ** Results shown are not statistically significant Low Risk / Low Dose Low Risk / High Dose High Risk / Low Dose High Risk / High Dose 17 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL
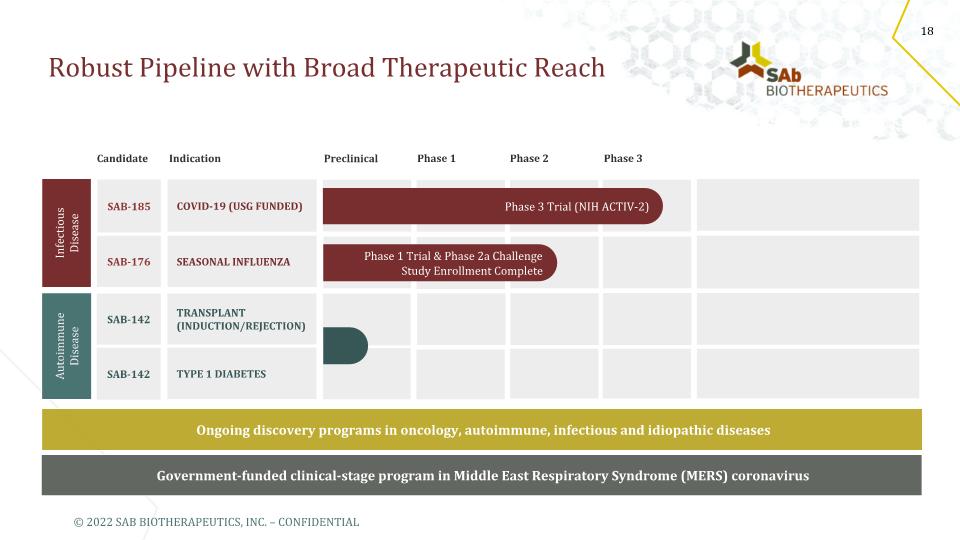
Robust Pipeline with Broad Therapeutic Reach Ongoing discovery programs in oncology, autoimmune, infectious and idiopathic diseases Government-funded clinical-stage program in Middle East Respiratory Syndrome (MERS) coronavirus SAB-176 SEASONAL INFLUENZA SAB-185 COVID-19 (USG FUNDED) SAB-142 SAB-142 TRANSPLANT (INDUCTION/REJECTION) TYPE 1 DIABETES Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 Phase 1 Trial & Phase 2a Challenge Study Enrollment Complete Autoimmune Disease Infectious Disease Phase 3 Trial (NIH ACTIV-2) © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Tc Goats™ - Expanding The Human Immunotherapeutic Platform for Personalized Medicine

Genetic Engineering Science Applied Across SAB Capra, LLC. is a wholly-owned subsidiary of SAB Biotherapeutics, Inc. Advancing novel antibody production platform leveraging transgenic goats Functionality of the HAC proven in a second species (ruminant ungulate) Generated H7N9-specific human polyclonal antibodies from Tc Goat (caprine) platform. Scientific Reports, 2019 SAB Capra Phase 2 STTR Grant (NIH/NIAID): in collaboration with Utah State University Total funding $1,501,157 ($926,194 to SABC, $574,963 to USU) Two years: 18 Apr 2019 – 31 Mar 2021 Two times of 12-month no cost extension granted—new end date 31 Mar 2023 Genetic optimization in our Tc Bovine was done in 10 years while the goat optimization was done in 2 years. 20 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Demonstrated Fully Human IgG in Tc Goat 21 Human IgG in Tc Capra Kids © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

High-dose therapy resulted in improved clinical parameters associated with reduced M. hominis burden following two subsequent infections JARED N SILVER, CAMERON D ASHBAUGH, JACOB J MILES, HUA WU, GREGORY T MARECKI, JOYCE K HWANG, JIN-AN JIAO, MARK ABRAMS, EDDIE J SULLIVAN, DUANE R WESEMANN, DEPLOYMENT OF TRANSCHROMOSOMAL BOVINE FOR PERSONALIZED ANTIMICROBIAL THERAPY, CLINICAL INFECTIOUS DISEASES, VOLUME 66, ISSUE 7, 1 APRIL 2018, PAGES 1116–1119 Confirms Feasibility of Multi-dosing Open wound persisted ~7 years prior to treatment Same area following treatment with SAB -136 Positioned for Personalized Medicine 22 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Advancing the Tc Platform for Continued Advancement of Human Health Developing targeted human polyclonal antibodies for use in personalized medicine Tc Goat platform production ready for producing diagnostics and testing reagent applications. Accommodating smaller volume markets, lower cost of development and maintenance, and accelerated scaling (shorter gestation, multiple births) 23 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL
