UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
|||
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On July 5, 2022, SAB Biotherapeutics, Inc. (the “Company” or “SAB”) made available a new corporate strategy presentation (the "Presentation") on the Investor Relations section of the Company’s website. A copy of the Presentation is furnished herewith as Exhibit 99.1 and is incorporated herein by reference.
The foregoing (including Exhibit 99.1) is being furnished pursuant to Item 7.01 and will not be deemed to be filed for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise be subject to the liabilities of that section, nor will it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act. The information contained in the Presentation is summary information that should be considered in the context of the Company’s filings with the Securities and Exchange Commission and other public announcements the Company may make by press release or otherwise from time to time.
Cautionary Note Regarding Forward-Looking Statements
Certain statements made in this Current Report on Form 8-K and the Presentation that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, including the development and efficacy of SAB-195 (C. Diff), SAB-176 (Influenza), SAB-142 (Type 1 Diabetes & Immunology), SAB-185 (COVID-19), and our other discovery programs; our cash runway into 2023; and potential future government and third-party collaborations or funded programs. These statements are based on the current expectations of SAB and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on, by any investor as a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict, may differ from assumptions, and are beyond the control of SAB. A further description of risks and uncertainties can be found in the sections entitled “Risk Factors” in SAB’s most recent Annual Report on Form 10-K, most recent quarterly reports on Form 10-Q, and in other filings SAB makes with the Securities and Exchange Commission, available at https://www.sec.gov/. Except as otherwise required by law, SAB disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits. The exhibits listed on the Exhibit Index are incorporated herein by reference.
Exhibit Number |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File-the cover page XBRL tags are embedded within the Inline XBRL document. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
SAB Biotherapeutics, Inc. |
|
|
|
|
Date: |
July 5, 2022 |
By: |
/s/ Eddie J. Sullivan |
|
|
|
Eddie J. Sullivan |

ADVANCING POWERFUL NEW CLASS OF IMMUNOTHERAPEUTIC ANTIBODIES July 2022 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL EXHIBIT 99.1

Forward Looking Statements 2 The material in this presentation has been prepared by SAB Biotherapeutics, Inc. (“SAB”) and is general background information about SAB’s activities current as of the date of this presentation. This information is given in summary form and is not intended to be complete. Information in this presentation, including financial forecasts, should not be considered advice or a recommendation to investors or potential investors in relation to holding, purchasing or selling securities or other financial products or instruments and does not take into account any particular investment objectives, financial situation or needs. This presentation may contain forward looking statements including statements regarding our intent, belief or current expectations with respect to SAB’s businesses and operations, market conditions, results of operations and financial condition, capital adequacy, specific provisions and risk management practices. Readers are cautioned not to place undue reliance on these forward-looking statements. SAB does not undertake any obligation to update any information herein for any reason or to publicly release the result of any revisions to these forward-looking statements to reflect events or circumstances after the date hereof to reflect the occurrence of unanticipated events unless required by law. While due care has been used in the preparation of forecast information, actual results may vary in a materially positive or negative manner and the presentation may contain errors or omissions. Forecasts and hypothetical examples are subject to uncertainty and contingencies outside SAB’s control. Past performance is not a reliable indication of future performance. The forward looking statements contained or implied in this presentation are subject to other risks and uncertainties, including those discussed under the heading "Risk Factors" in SAB’s most recent Annual Report on Form 10-K with the Securities and Exchange Commission (the “SEC”) and in other filings that SAB makes with the SEC. Unless otherwise specified, information is current at the date hereof. The SAB logo and other trademarks of SAB appearing in this presentation are the property of SAB. All other trademarks, services marks, and trade names in this presentation are the property of their respective owners. © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Experienced Management Team Christoph Bausch, PhD, MBA CHIEF OPERATING OFFICER 15+ years platform technology commercialization Sigma Aldrich Stowers Institute Postdoc Eddie J. Sullivan, PhD PRESIDENT & CEO / CO-FOUNDER 20 years new technology development 25+ years biotech Former Japanese pharma BIO Executive Committee Reproductive physiologist Russell Beyer, MBA, CMA CHIEF FINANCIAL OFFICER 25+ years Pharma & Fortune 100 Country/region CFO at AstraZeneca, Clorox Track record of driving growth, integrations Strategic financial, operations, reporting, planning Samuel J. Reich EXECUTIVE CHAIRMAN, BOD 20 years Biopharma Executive and BOD Bioentrepreneur Co-founder Acuity Pharmaceuticals, OPKO Health, Biscayne Neurotherapeutics Molecular Biologist, Inventor, former PENN © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL Alexandra Kropotova, MD CHIEF MEDICAL OFFICER 20+ years global clinical development Biopharmaceutical R&D leader, Pfizer, Wyeth, Sanofi, Teva Specialty R&D Board member, iBio Contributed to numerous patents & compounds leading portfolios from Phase I to BLA and NDA approvals

Novel DiversitAb™ Platform for Developing Highly-Differentiated Immunotherapies Vertical integration enables rapid, scalable development of multi-targeted products Robust, growing clinical-stage pipeline spanning multiple therapeutic areas Established proof-of-concept through US Government funded programs & partnerships totaling ~$200MM Strong corporate position with experienced leadership team and growing infrastructure Leveraged advanced genetic engineering & antibody science to develop Tc bovine-derived fully-human polyclonal antibodies © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL Innovative DiversitAb™ platform produces a new class of targeted fully-human, highly-potent polyclonal antibodies
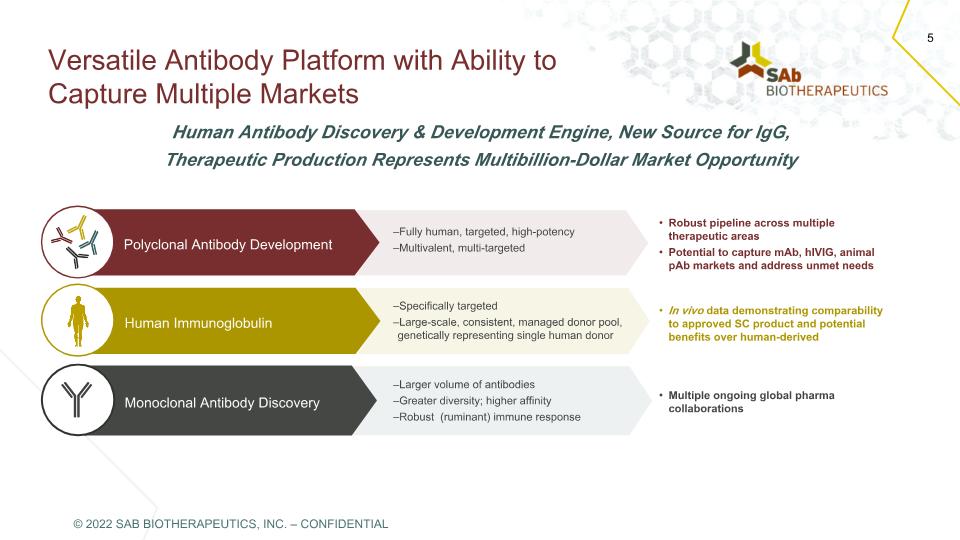
Versatile Antibody Platform with Ability to Capture Multiple Markets 5 Human Antibody Discovery & Development Engine, New Source for IgG, Therapeutic Production Represents Multibillion-Dollar Market Opportunity Monoclonal Antibody Discovery –Larger volume of antibodies –Greater diversity; higher affinity –Robust (ruminant) immune response –Fully human, targeted, high-potency –Multivalent, multi-targeted Human Immunoglobulin –Specifically targeted –Large-scale, consistent, managed donor pool, genetically representing single human donor Multiple ongoing global pharma collaborations Polyclonal Antibody Development In vivo data demonstrating comparability to approved SC product and potential benefits over human-derived Robust pipeline across multiple therapeutic areas Potential to capture mAb, hIVIG, animal pAb markets and address unmet needs © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL
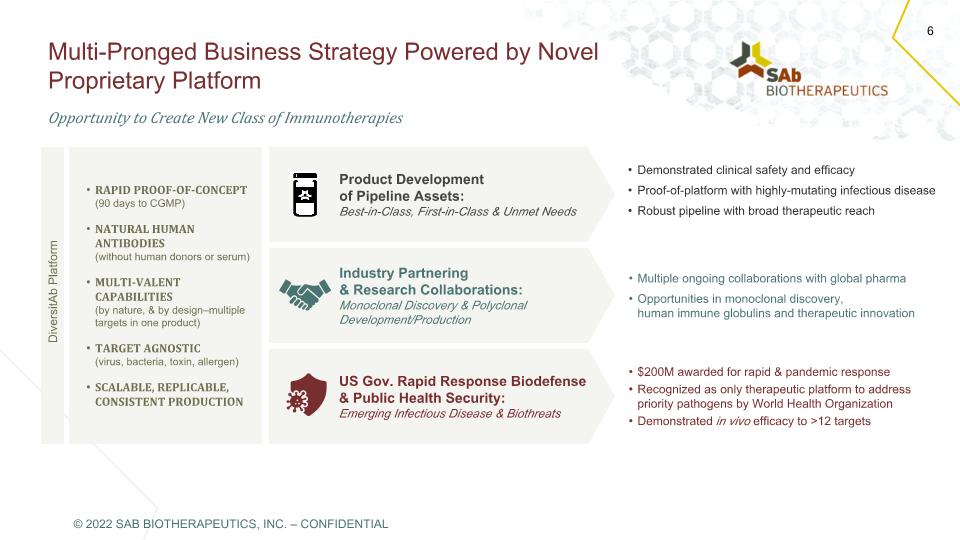
Product Development of Pipeline Assets: Best-in-Class, First-in-Class & Unmet Needs Industry Partnering & Research Collaborations: Monoclonal Discovery & Polyclonal Development/Production US Gov. Rapid Response Biodefense & Public Health Security: Emerging Infectious Disease & Biothreats Multi-Pronged Business Strategy Powered by Novel Proprietary Platform 6 $200M awarded for rapid & pandemic response Recognized as only therapeutic platform to address priority pathogens by World Health Organization Demonstrated in vivo efficacy to >12 targets RAPID PROOF-OF-CONCEPT (90 days to CGMP) NATURAL HUMAN ANTIBODIES (without human donors or serum) MULTI-VALENT CAPABILITIES (by nature, & by design–multiple targets in one product) TARGET AGNOSTIC (virus, bacteria, toxin, allergen) SCALABLE, REPLICABLE, CONSISTENT PRODUCTION Demonstrated clinical safety and efficacy Proof-of-platform with highly-mutating infectious disease Robust pipeline with broad therapeutic reach Multiple ongoing collaborations with global pharma Opportunities in monoclonal discovery, human immune globulins and therapeutic innovation Opportunity to Create New Class of Immunotherapies DiversitAb Platform © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

DiversitAb™ Platform Advancing a new class of fully-human polyclonal Tc bovine-derived antibodies without the need for human serum ANTIGEN PATHOGEN TC BOVINE™ HYPERIMMUNIZATION HUMAN ANTIBODIES PURIFICATION TARGETED HIGH-POTENCY IMMUNOTHERAPY Reliable, controlled, consistent production of diverse, high-titer, high-avidity, fully-human polyclonal antibodies Generated antibodies behave similarly to human-derived with ability to specifically target Proprietary immunization strategies and robust immune response drive extremely high potency Well-established and understood regulatory path as biologic through FDA-CBER Vertical integration enabling rapid, scalable development and production of multivalent products © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

FDA: CENTER FOR DRUG EVALUATION & RESEARCH (CDER) FDA: CENTER FOR BIOLOGICS EVALUATION & RESEARCH (CBER) pAbs TARGET Monoclonal Approach Highly-targeted with specific activity Iterative Ab identification and selection process Selected and cloned in vitro May promote escape mutants via selective pressure Resistance may develop as pathogen/target mutates Current cocktail trend to address resistance Polyclonal Approach Diversity of antibodies with multiple modalities Naturally selected and produced in vivo Effective against escape mutants Reduced possibility of resistance Activates cellular immunity Synergistic properties not duplicated by mono- or oligoclonals mAb TARGET Clones of a single antibody bind to a specific epitope Natural mixture of many antibodies bind to multiple epitopes Characterized Monoclonal Antibody Plasma-Derived Polyclonal Antibodies Polyclonals: Broader Spectrum Efficacy Valuable in Range of Indications 8 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

High-dose therapy resulted in improved clinical parameters associated with reduced M. hominis burden following two subsequent infections JARED N SILVER, CAMERON D ASHBAUGH, JACOB J MILES, HUA WU, GREGORY T MARECKI, JOYCE K HWANG, JIN-AN JIAO, MARK ABRAMS, EDDIE J SULLIVAN, DUANE R WESEMANN, DEPLOYMENT OF TRANSCHROMOSOMAL BOVINE FOR PERSONALIZED ANTIMICROBIAL THERAPY, CLINICAL INFECTIOUS DISEASES, VOLUME 66, ISSUE 7, 1 APRIL 2018, PAGES 1116–1119 Open wound persisted ~7 years prior to treatment Same area following treatment with SAB -136 Demonstrated Human Safety and Efficacy in Multi-Dosing Regimen 9 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

DiversitAb™ Platform is Clinically Validated Across Several Targets Referenced Trials: Safety, Tolerability, and Pharmacokinetics of SAB-176 in Healthy Participants – Full Text View - ClinicalTrials.gov Study of SAB-176 in Healthy Adult Participants - Full Text View - ClinicalTrials.gov Safety, Tolerability, and Pharmacokinetics of SAB-185 in Healthy Participants – Full Text View - ClinicalTrials.gov Safety, Tolerability, and Pharmacokinetics of SAB-185 in Ambulatory Participants With COVID-19 - Full Text View - ClinicalTrials.gov ACTIV-2: A Study for Outpatients With COVID-19 - Full Text View - ClinicalTrials.gov Safety, Tolerability, and Pharmacokinetics of SAB-301 in Healthy Adults – Full Text View - ClinicalTrials.gov Public Collaborations DoD, BARDA, NIH NIAID, Naval Medical Research Center, USAMRIID Filed in US and ex-US 7 Clinical Trials Span from Phase 1 to Phase 3 across 3 indications 3INDs & 1 CTA Academic Collaborations Brigham and Women’s Hospital, Harvard, University of South Dakota, University of Pittsburgh 10 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Intellectual Property © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL 11

Intellectual Property © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL 12

Scaled Infrastructure & Capacity: Tc Bovine & Plasma Production Facility 13 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Scaled Infrastructure & Capacity: Laboratory & Manufacturing Manufacturing Facility (200L) Plasma Purification Suite (50L) 14 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL CET Lab Labeling Suite Fill Suite Cell Culture Lab 50L Suite

SELECTED PIPELINE PROGRAMS

Robust Biologic Pipeline with Broad Polyclonal Therapeutic Reach © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Clinical Development Programs: Focus on the Next 4+ Years Consistent delivery of one IND per year SAB-195 IND (C. diff.) SAB-176 2023-2024 flu season Phase 2b FPI SAB-142 IND (T1D) SAB-142 Phase 1/POBA in T1D early onset FPI SAB-195 Phase 1/POBA FPI SAB-195 Phase 2 FPI SAB-176 2023-2024 flu/COVID season Phase 2b topline SAB-142 IND (immunology) SAB-142 Phase 1/POBA T1D early onset ongoing SAB-195 Phase 2 Top line SAB-176 Phase 3 FPI SAB-142 Phase 1/POBA T1D early onset top line SAB-195 Phase 3 start 2023 2024 2025 2026 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-195: Clostridioides difficile Infections - Fast to Proof of Concept Option © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Clostridioides difficile Infection (CDI or C. diff.) is a bacterial infection of the large intestine (colon). A spectrum of clinical disease ranges from mild diarrhea to severe. CDI is characterized by abdominal pain, fever, diarrhea, nausea, and vomiting. Complications of severe CDI include kidney failure, toxic megacolon, bowel perforation, and death. CDI infection is one of the most prevalent health care–associated bacterial infections in the US and developed world ~ 500,000 infections per year in the US1 ~ 30,000 death in the US1 CDI infection is associated with significant costs: Up to $4.8 billion each year in excess health care costs for acute care facilities alone1 Patients with the first CDI recurrence have a risk of subsequent recurrence from 25% to 40% and higher1, 2 CDI-attributable median length of stay and costs (in US$) increased from 7 (4-13) days and $13,168 ($7,525-$24,456) for patients with primary CDI only to 15 (8-25) days and $28,218 ($15,050-$47,030) for patients with recurrent CDI2 The risk of death for patients with recurrent CDI is 33% higher compared to those patients without recurrence High Unmet Medical Needs Remain References: 1. CDC. Atlanta, GA: U.S. Department of Health and Human Services. Accessed 6/27/2022 Nearly half a million Americans suffered from Clostridium difficile infections in a single year | CDC Online Newsroom | CDC 2. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study . J Hosp Infection. 2016 Jul;93(3):286-9 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL High Morbidity, Mortality, and Costs

Value Proposition: SAB-195 Key Differentiators First in class fully human polyclonal antibody treatment with dual mechanism of action designed to treat severe CDI and reduce CDI recurrence in high-risk patients © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL First in class fully human polyclonal antibody treatment Only treatment with dual mode of action: Unlike bezlotoxumab, SAB-195 targets surface antigen on C. difficile as well as multiple toxins Unlike antibiotics, SAB-195 targets several C. difficile toxins responsible for severity of the disease SAB-195 is target-specific treatment targeting only C. difficile while fully preserving good microbiome Preclinical data supports potential for competitive efficacy as first-line pAb therapy for severe CDI in patients who are at high risk for CDI recurrences

SAB-195 Preclinical Data Tc bovine Immunized with Antigen Fusion Proteins Constructed from RBD of TcdA,TcdB(630), TcdB(027) and CDT Tc bovine-derived anti-quadrivalent toxin hIgG provided 90% to 100% protection in hamsters against C. difficile strain 630 or more virulent epidemic strain NAP1 Clostridium difficile chimeric toxin receptor binding domain vaccine induced protection against different strains in active and passive challenge models.. Jing-Hui Tian a, Gregory Glenn a, David Flyer a, Bin Zhou a, Ye Liu a, Eddie Sullivan b, Hua Wub, James F. Cummings a, Larry Ellingsworth a,⇑, Gale Smith https://pubmed.ncbi.nlm.nih.gov/28669616/#:~:text=Vaccine,33)%3A4079%2D4087 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-195 Development Timelines Pre-Clinical/ IND Enabling IND Filing Phase I in HVs Top Line Data Phase 1 POBA Phase 2a/b in Target Patient Population Phase 2b Top Line 2022-2023 H2 2023 -H1 2024 2024 2024 2024 - 2025 H2 2025 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-176: First In Class Biologic Anti-Influenza Treatment © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Unmet Need of Seasonal Influenza Devastating health and economic impacts Estimated 30,000 - 50,000 deaths/year U.S. with 290,000 - 650,000 globally ~500,000 hospitalizations annually in U.S. Average US hospital stay: $8,000 - $9,000/day; 4-8 days/stay Often 30% - 70% failure rate for vaccine; vaccine ineffective in at-risk sub-populations No current effective treatment for seasonal influenza Current antiviral has a 48-hour window Approved antiviral small molecule treatments may shorten duration of fever and symptoms, but not effective against clinically meaningful endpoints or neuraminidase mutation; limited efficacious window 35,500,000 ILLNESSES 34,200 DEATHS 1 of 1,000 INFECTIONS RESULTED IN DEATH CDC; 2018-19 FLU SEASON © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Value Proposition: SAB-176 Key Differentiators First in class fully human polyclonal antibody treatment aimed to provide superior long-lasting efficacy for prophylaxis and management of influenza in patients at high risk © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL First and only biologic for management of influenza in high-risk patients Adaptive and cross-reactive to multiple influenza strains Fully human pAbs uniquely positioned to manage influenza course in high-risk patients including but not limited to: Immunocompromised Immunosenescent patients Patients in long-term care facilities Established Proof of Concept in the well-established validated influenza challenge model

B/Florida/04/2006 Challenge strain SOURCE: NEXTFLU AT HTTPS://NEXTFLU.ORG/VIC/12Y/ Antibodies produced to B/Phuket/3073/2013 protected against B/Florida/04/2006 2006 2017 B/Phuket/3073/2013 Vaccination strain Highly-Mutational Influenza Virus BYAM PHYLOGENIC TREE 100% Protection at All Dose Levels in Influenza Mouse Challenge * x Adaptive & Cross Reactive to Mutating Strains Efficacy Against Mutational Drift 26 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Established Proof of Concept for SAB-176: Met Primary Endpoint of Viral Load Reduction in Phase 2a Challenge Study Achieved Statistically Significant (p = 0.026) Reduction in Viral Load Mean Viral Load by Nasal Samples qRT-qPCR by Day Relative to Viral Challenge 27 Mean Total Symptom Score by Day Relative to Viral Challenge SAB-176 Achieved Statistically Significant (p = 0.013) Improvement in Symptomology at Day 4 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL SAB-176 not specifically targeted to pH1N1 strain used in challenge study Statistically significant reduction in virus load confirms high cross reactivity to pandemic strain (not targeted with immunogen) Reinforces ability to generate broadly neutralizing antibodies to viral variants
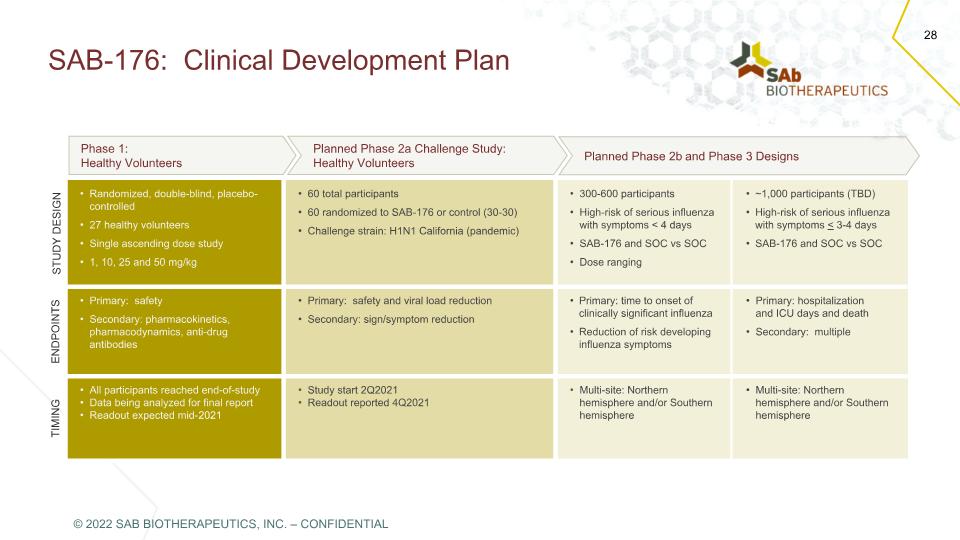
SAB-176: Clinical Development Plan Phase 1: Healthy Volunteers Planned Phase 2a Challenge Study: Healthy Volunteers Planned Phase 2b and Phase 3 Designs STUDY DESIGN Randomized, double-blind, placebo-controlled 27 healthy volunteers Single ascending dose study 1, 10, 25 and 50 mg/kg 60 total participants 60 randomized to SAB-176 or control (30-30) Challenge strain: H1N1 California (pandemic) 300-600 participants High-risk of serious influenza with symptoms < 4 days SAB-176 and SOC vs SOC Dose ranging ~1,000 participants (TBD) High-risk of serious influenza with symptoms < 3-4 days SAB-176 and SOC vs SOC ENDPOINTS Primary: safety Secondary: pharmacokinetics, pharmacodynamics, anti-drug antibodies Primary: safety and viral load reduction Secondary: sign/symptom reduction Primary: time to onset of clinically significant influenza Reduction of risk developing influenza symptoms Primary: hospitalization and ICU days and death Secondary: multiple TIMING All participants reached end-of-study Data being analyzed for final report Readout expected mid-2021 Study start 2Q2021 Readout reported 4Q2021 Multi-site: Northern hemisphere and/or Southern hemisphere Multi-site: Northern hemisphere and/or Southern hemisphere © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-176 Development Timelines Study Startup Activities Phase 2b Study First Patient In Phase 2b Study Ongoing Phase 2b Top Line Results Phase 3 Study First Patient In 2022-2023 Q4 2023 2023-2024 Influenza Season 2024 2025 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-142: Asset with a Multi-Indication Potential © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Disease-modifying treatments in late-stage development: >100 active interventional trials with small molecules, biologics, and cell therapies in Type 1 Diabetes Type 1 Diabetes High Unmet Medical Needs Drive High Level of Competition © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Value Proposition: SAB-142 Key Differentiators First in class fully human polyclonal antibody treatment aimed to provide superior efficacy for delaying onset of clinical Stage 3 T1D © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL First in class fully human polyclonal antibody treatment aimed to provide superior efficacy for delaying onset of clinical Stage 3 T1D Validated Mechanism of Action by a 3rd party ATG demonstrating reduction in decline in C-peptide vs. placebo (Haller, 2019)
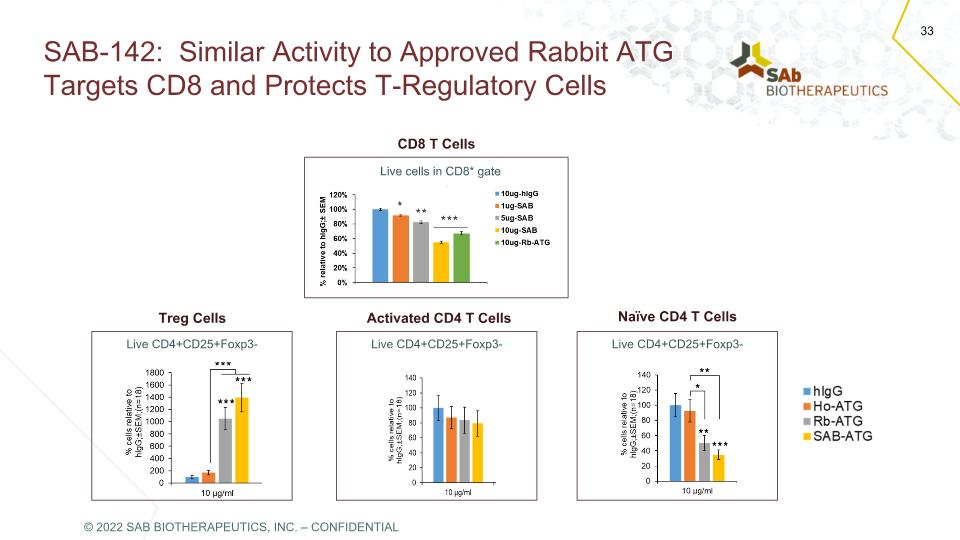
SAB-142: Similar Activity to Approved Rabbit ATG Targets CD8 and Protects T-Regulatory Cells CD8 T Cells Treg Cells Live cells in CD8* gate Live CD4+CD25+Foxp3- Activated CD4 T Cells Live CD4+CD25+Foxp3- Naïve CD4 T Cells Live CD4+CD25+Foxp3- © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-142 Pre-clinical Data Continued Major subsets of peripheral blood lymphocytes Figure. Changes in major subsets of peripheral blood lymphocytes (total lymphocytes. T and B cells, CD4+ and CD8+ T helpers and killers, respectively) following SAB-142 and ATG treatments. Red: 5 mg/kg ATG; Blue: 1 mg/kg SAB-142; Grey: 5 mg/kg SAB-142 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-142: MoA Clinically Validated by 3rd Party Compound 35 p=0.00005 ATG vs Placebo Placebo *RABBIT ATG FROM SANOFI – NOT SAB-142 (HUMAN TC-BOVINE DERIVED ATG) Haller et al. Diabetes. 2019. June, 68(6): 1267-1276 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL EQUINE ATGAM-AF488 Tc Bovine Human-PB, Rabbit THYMO-AF488, Equine ATGAM-AF488 and Anti-CD3-APC RABBIT THYMO-AF488 2 Years: Low-Dose ATG* Preserved C-Peptide in New Onset T1D

SAB-142: Clinical Development Plan T1D Phase 1-2: Early Onset T1D in Adults, followed by adults and adolescents at C-peptide interim analysis Phase 3: New and Recent Onset T1D in Adults and Children (Study 1) At Risk Adults and Children (Study 2) STUDY DESIGN Open-label Teplizumab or ATG more likely to be a control XX participants Ascending dose SAB-142 study XXX mg/kg (pre-clinical NHP data will adjust) Biomarker-driven escalation with adaptive randomization based on Safety + CD4, CD8+ cells and Tregs Randomized, blinded, PBO and teplizumab controlled 90 (45:45), a control is either ATG or teplizumab SAB-142 vs ATG/ teplizumab ENDPOINTS Primary: acute and long-term safety Primary POBA: C-peptide Secondary: pharmacokinetics, pharmacodynamics, hypersensitivity (ADA), C-protein, HbA1c, T regs, CD3, CD8/CD4 and other markers. New and Recent Onset T1D in Adults and Children (Study 1): Primary: improvement/control of TID disease Secondary: safety, pharmacokinetics, pharmacodynamics, hypersensitivity and serum sickness (ADA), C-protein, HbA1c, CD3, CD8/CD4 and other markers. At Risk Adults and Children (study 2): Primary: time to onset of clinical stage (Stage 3) T1D Secondary: safety, pharmacokinetics, pharmacodynamics, hypersensitivity and serum sickness (ADA), C-protein, HbA1c, CD3, CD8/CD4 and other markers. © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

SAB-142 Development Timelines Pre-clinical / IND Enabling IND Filing Phase 1 / Proof of Biological Activity in Adult T1D Phase 1 / Proof of Biological Activity in Pediatric T1D Phase 1 / POBA Top Line 2022-2023 2024 2024 2025 2026 © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL

Summary 38 Executive Management: Proven team with biotech startup, rapid drug development, and entrepreneurial experience. Platform: Innovative DiversitAb™ platform produces a new class of targeted fully-human, highly-potent polyclonal antibodies, with a broad efficacy spectrum in a broad range of indications. SAB-195: Preclinical data supports potential for competitive efficacy as first-line pAb therapy for severe CDI in patients who are at a high risk for recurrences, expect to file IND in 2H 2022. SAB-176: First in class fully human polyclonal antibody treatment aimed to provide superior efficacy for prophylaxis and management of influenza in patients at high risk, planned initiation of Phase 2b trial in 2H 2023. SAB-142: First in class fully human polyclonal antibody treatment aimed to provide superior efficacy for delaying onset of clinical Stage 3 Type 1 Diabetes, IND submission expected in 2024. © 2022 SAB BIOTHERAPEUTICS, INC. – CONFIDENTIAL
